Nicotinamide mononucleotide (NMN) as an anti-aging health product – Promises and safety concerns
노화방지 건강제품으로서의 니코틴아미드 모노뉴클레오타이드(NMN) – 약속 및 안전 문제
Author links open overlay panelHarshaniNadeeshaniaJinyaoLibTianleiYingcBaohongZhangdJunLuaefghij
https://doi.org/10.1016/j.jare.2021.08.003Get rights and content
Under a Creative Commons license
open access
Abstract
Background 배경
세계적으로 고령화 인구가 점차 증가함에 따라 장수를 보장하고, 노화관련 합병증을 개선하기 위한 항노화 건강제품에 대한 수요도 증가하고 있습니다.
다양한 노화방지 건강제품 중 니코틴아미드 모노뉴클레오타이드(NMN)는 소비자와 과학계의 주목을 받고 있습니다.
Elderly population has been progressively rising in the world, thus the demand for anti-aging heath products to assure longevity as well as to ameliorate age-related complications is also on the rise. Among various anti-aging health products, nicotinamide mononucleotide (NMN) has been gaining attentions of the consumers and the scientific community.
Aim of review 검토의 목적
This article intends to provide an overview on the current knowledge on promises and safety concerns of NMN as an anti-aging health product.
이 글은 노화방지 건강제품으로서 NMN의 약속 및 안전 문제에 대한 현재 지식에 대한 개요를 제공하고자 합니다.
검토의 주요 과학적 개념
Key scientific concepts of review
우리 몸의 니코틴아미드 아데닌 디뉴클레오타이드(NAD+) 수치는 노화와 함께 고갈되며, 미토콘드리아의 에너지 생산 하향조절, 산화스트레스, DNA 손상, 인지장애, 염증질병과 관련이 있습니다.
그런데 NAD+의 전구체 NMN은 신체의 NAD+ 수준을 높여 이 과정을 늦출 수 있습니다.
많은 생체내 연구에서 NMN 보충으로 인한 다양한 연령유발 합병증에 대한 치료효과의 긍정적 결과가 나타났습니다.
NMN 투여의 안전성 문제를 조사하기 위해 1건의 임상전 및 1건의 임상연구가 수행되었으며 몇 가지 인간 임상시험이 추가로 수행되고 있습니다.
NMN기반 노화방지 제품이 시장에 대량 출시됨에 따라 NMN 보충제의 효과 및 안전성을 찾기 위한 적절한 임상조사가 시급합니다.
Nicotinamide adenine dinucleotide (NAD+) levels in the body deplete with aging and it is associated with downregulation of energy production in mitochondria, oxidative stress, DNA damage, cognitive impairment and inflammatory conditions. However, NMN, as the precursor of NAD+, can slow down this process by elevating NAD+ levels in the body. A number of in vivo studies have indicated affirmative results of therapeutic effects for various age-induced complications with NMN supplementation. One preclinical and one clinical study have been conducted to investigate the safety concerns of NMN administration while a few more human clinical trials are being conducted. As there is a large influx of NMN based anti-aging products on the market, proper clinical investigations are urgently needed to find out the effectiveness and safety of NMN supplementation.
Graphical abstract
Keywords
#Age-induced diseases #Anti-aging #Nicotinamide #adenine dinucleotide Nicotinamide #mononucleotide #Supplement #노화방지
#노화방지보충제 #노화예방보충제
Introduction 서문
20세기에 전염병이 성공적으로 통제되면서, 많은 국가에서 평균 수명이 급격히 증가했습니다.
2019년 전 세계 65세 이상 인구는 7억 290만 명에서 2050년에는 1억 5489만 명으로 증가할 것으로 예상됩니다[1].
2019년 전 세계 65세 이상 인구의 비율과 중변량 전망에 따른 향후 전망을 그림 1에 나타냈습니다.
The successful control of communicable diseases in the 20th century led to a sharp rise in the mean life expectancy of many countries. In 2019, number of persons, aged 65 or over was 702.9 million in the world and it is projected to be 1548.9 million by 2050 [1]. Percentage of global population aged 65 years or over in 2019 and future projections according to the medium-variant projection is illustrated in Fig. 1.
고령인구 증가와 함께,
죽상동맥경화증, 고혈압, 골관절염, 알츠하이머병, 파킨슨병, 당뇨병, 암을 포함한, 신경퇴행성 질병 등 노화관련 질병의 유병률
이 증가하면서 전 세계적으로 사회경제적, 의료적 부담이 가중되고 있습니다[2].
Along with increasing elderly population, the prevalence of age-related diseases such as atherosclerosis, hypertension, osteoarthritis, neurodegenerative diseases including Alzheimer’s and Parkinson’s diseases, diabetes mellitus and cancers has gone up leading to heavy global socioeconomic and medical burden [2].

Download : Download high-res image (60KB)Download : Download full-size image
Fig. 1. Percentage of global population aged 65 years or over according to the medium-variant projection (UN, 2019).
따라서 노화영향을 완화하기 위해, 영양보조제, 다양한 약물, 운동 프로그램, 호르몬요법, 기타 치료법을 권장하는 노인관리 의료관행이 세계적으로 급증하고 있습니다.
이에 따라 안티에이징 건강제품에 대한 소비자 수요와 글로벌 시장가치가 상승하고 있습니다[3].
소비자의 과도한 수요와 제조업체의 높은 이윤이 적절한 안전성 테스트 없이 출시되는 안티에이징 건강 제품의 주요 원동력입니다[4].
따라서 신중하고 포괄적이고 단계적인 과학적 임상전 및 임상조사를 수행하는 것이 중요합니다.
Therefore, age management medical practices have been mushrooming in the world for recommending nutritional supplements, various drugs, exercise programs, hormone therapies and other treatments to mitigate the effect of aging. Consequently, the consumer demand and the global market value for anti-aging health products are on the rise [3]. Excessive demand of consumers and high profit margin for manufacturers are the major driving force behind the release of anti-aging health products without adequate safety testing [4]. Thus, careful comprehensive and stepwise scientific preclinical and clinical investigations are crucial to be conducted.
다양한 안티에이징 건강제품 중 니코틴아미드 모노뉴클레오타이드(NMN)는 유망한 안티에이징 제품으로 주목받고 있습니다.
노화의 원인이 되는 미토콘드리아 붕괴는 체내 니코틴아미드 아데닌 디뉴클레오티드(NAD+) 수치가 증가하면 되돌릴 수 있습니다. NMN은 NAD+ 생합성의 중간체 역할을 하는 NAD+의 전구체이며, NMN의 식이보충제는 체내 NAD+ 수준을 증가시키는 것으로 밝혀졌습니다[5].
NMN은 nicotinamide와 ribose를 포함하는 nucleoside와 인산기가 반응하여 형성된 생리활성 뉴클레오티드입니다[6].
다양한 식물 및 동물성 식품 공급원에 자연적으로 존재합니다.
또한, 생명공학적 생산 및 박테리아 및 효모로부터 NMN 정제 가능성을 조사하기 위한 여러 연구가 수행되었습니다[7].
Among various anti-aging health products, nicotinamide mononucleotide (NMN) has been gaining an increasing attention as a promising anti-aging product. The mitochondrial decay, which is responsible for aging, can be reversed by the increased levels of nicotinamide adenine dinucleotide (NAD+) in the body. NMN is a precursor of NAD+ that acts as an intermediate in NAD+ biosynthesis, while dietary supplements of NMN are found to increase the NAD+ levels in the body [5]. NMN is a bioactive nucleotide formed by the reaction between a nucleoside comprising nicotinamide and ribose and a phosphate group [6]. It naturally presents in a variety of plant and animal food sources. Furthermore, several studies have been carried out to investigate the potential of biotechnological production and purifying NMN from bacterial and yeasts [7].
NMN의 노화방지 가능성 외에도 다양한 생체내 연구에서 광범위한 약리학적 활성이 확인되었습니다.
Other than anti-aging potential of NMN, a wide range of pharmacological activities have been identified in a number of in vivo studies.
The link between NMN and the incidence of Alzheimer’s disease, obesity and associated complications, cerebral and cardiac ischemia, and age- and diet-induced type 2 diabetes has been studied extensively [8].
NMN과 알츠하이머병, 비만 및 관련 합병증, 뇌 및 심장 허혈, 연령 및 식이 유발성 제2형 당뇨병의 발병률 사이의 연관성이 광범위하게 연구되었습니다[8].
이전에는 NMN이 NAD+ 생합성 중간체로서만 과학계 주목을 받았지만, 최근에는 체내 NAD+ 수치 증가에 의해 유발되는 여러 약리학적 활성, 특히 노화방지 활성이 주목받고 있습니다.
Though, previous attention of scientific community has been paid on NMN only as an intermediate in NAD+ biosynthesis, recently, a number of pharmacological activities triggered by increasing NAD+ levels in the body, especially anti-aging activity have been taken the centre of attention.
결과적으로, 세포배양, 동물모델 및 인간 임상시험 등 많은 연구가 노화방지 건강제품으로 NMN을 사용하는 것에 대한 약속과 안전성 문제, 그리고 노화관련 질병예방을 위한 보충제로 NMN을 사용할 가능성을 조사하기 위해 수행되었습니다.
As a result, a number of studies including cell culture, animal models and human clinical trials have been conducted to investigate the promises and the safety concerns of using NMN as an anti-aging health product and the potential of using NMN as a supplement to avoid age-related disease conditions.
따라서 이 리뷰는 노화방지 건강제품으로 NMN을 사용하는 것에 대한 약속과 안전성 문제, 다른 약리학적 및 치료적 용도 및 노화방지 특성의 기초가 되는 작용 메커니즘에 대한 가장 최근의 발전과 현재 지식을 제시하고자 하며,
추가 연구를 자극하고, NMN의 성공적 임상전 및 임상 항노화 결과를 노화 및 노화관련 질병의 효과적 치료로 전환할 가능성에 대한 통찰력을 제공하는 데 관심이 있습니다.
Hence, this review intends to present the most recent advances and current knowledge on promises and safety concerns of the use of NMN as an anti-aging health product, its other pharmacological and therapeutic uses and mechanism of action underlying the anti-aging properties with an interest to stimulate further research and offer an insight to the possibility of translating successful preclinical and clinical anti-aging outcomes of NMN into an effective treatment of aging and age-related diseases.
What is nicotinamide mononucleotide (NMN)?
니코틴아마이드 모노뉴클레오타이드(NMN)란 무엇입니까?
니코틴아미드 모노뉴클레오타이드(NMN)는 α 및 β 아노머 형태로 존재하며,
니코틴아미드 리보타이드, 니코틴아미드-1-ium-1-β-D-리보푸라노사이드 5'-포스페이트, β-니코틴아미드 리보스 모노포스페이트 및 3-카바모일-1-[5-O-(히드록시포스피나토)-β-D-리보푸라노실] 피리디늄으로 식별됩니다[9].
Nicotinamide mononucleotide (NMN) exists as α and β anomeric forms while it is identified as nicotinamide ribotide, nicotinamide-1-ium-1-β-D-ribofuranoside 5′-phosphate, β-nicotinamide ribose monophosphate and 3-carbamoyl-1-[5-O-(hydroxyphosphinato)-β-D-ribofuranosyl] pyridinium, among others [9].
β 형태는 활성 아노머이며, NMN은 니코틴아미드(비타민 B3의 아미드 형태) 및 리보스를 포함하는 뉴클레오시드와 인산기와 사이에서 니코틴아미드 포스포리보실트랜스퍼라제 효소에 의해 촉매되는 반응의 결과로 자연적으로 구조화됩니다[10].
The β form is the active anomer and NMN is naturally structured as a result of a reaction, which catalysed by nicotinamide phosphoribosyltransferase enzyme, between a phosphate group and a nucleoside comprising nicotinamide (an amide form of vitamin B3) and ribose [10].
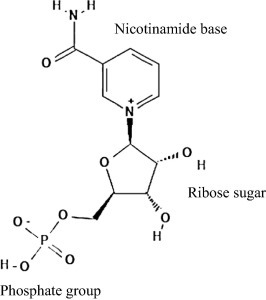
NMN은 피리딘 염기를 가진 생리활성 뉴클레오타이드이며, 분자량은 334.22g/mol입니다.
상당히 산성이며 수용성 화합물입니다.
용해도는 1.8 mg/mL로 보고되었습니다[11](그림2).
NMN is a bioactive nucleotide with a pyridine base and its molecular weight is 334.22 g/mol.
It is fairly acidic and water-soluble compound.
The solubility has been reported to be 1.8 mg/mL [11] (Fig. 2).
Download : Download high-res image (84KB)Download : Download full-size image
Fig. 2. Chemical structure of nicotinamide mononucleotide (NMN).
NMN은 주로 핵, 미토콘드리아 및 세포질에 위치하는 반면, 인체에서는 태반조직 및 혈액 및 소변과 같은 체액에서 발견될 수 있습니다[9].
그것은 미숙한 콩 꼬투리, 양배추, 오이, 브로콜리, 토마토, 버섯, 아보카도 등 다양한 과일과 채소는 물론 생 쇠고기와 새우에서 자연적으로 발견됩니다.
NMN is mainly located in the nucleus, mitochondria and cytoplasm, whereas in the human body, it can be found in placenta tissue and body fluids such as blood and urine [9].
It is naturally found in a variety of fruits and vegetables including immature soybean pods, cabbage, cucumber, broccoli, tomato, mushroom and avocado as well as in raw beef and shrimp.
이러한 야채와 과일의 NMN 함량은 각각 0.25–1.88 mg/100 g 및 0.26–1.60 mg/100 g인 반면, 생 쇠고기와 새우는 식물성 식품(0.06–0.42 mg/100 g)보다 상대적으로 낮은 수준의 NMN을 함유하고 있습니다.
The NMN content in these vegetables and fruits are 0.25–1.88 mg/100 g and 0.26–1.60 mg/100 g, respectively, whereas raw beef and shrimp contain comparatively lower level of NMN than those plant-based food (0.06–0.42 mg/100 g).
적혈구에 NMN이 존재한다는 증거를 바탕으로 NAD+의 생합성 및 많은 생리적 기능에 필요한 생리학적으로 적절한 NMN 함량이 일상적 식품 공급원에서 흡수된다는 주장이 제기되었습니다[10].
Based on the evidence of the presence of NMN in red blood cells, it has been put forward that physiologically pertinent NMN contents, which are required for the biosynthesis of NAD+ and many physiological functions, are absorbed from daily food sources [10].
NMN은 NAD+ 생합성의 중간체입니다.
NMN is an intermediate of NAD+ biosynthesis.
NAD+ is a very important metabolic redox co-enzyme in eukaryotic organisms and is essential component for large number of enzymatic reactions.
NAD+는 진핵생물에서 매우 중요한 대사 산화환원 조효소이며, 많은 효소 반응의 필수 성분입니다.
그것은 세포사멸, 노화, 유전자 발현, 신경염증, DNA 복구 등 신체의 다양한 생물학적 과정에서 중요한 역할을 하며, 이는 NAD+가 인간 수명과 건강에 중요한 역할을 함을 나타냅니다[12].
It plays a vital role in a variety of biological processes of the body including cell death, aging, gene expression, neuroinflammation and DNA repair, which indicating a significance role of NAD+ in longevity and health of human life [12].
많은 최근 연구에서 밝혀진 바와 같이, NAD+ 결핍은 다양한 질병 상태에서 다양한 약리학적 활성에 영향을 미치는 NMN 보충으로 보상될 수 있습니다[13].
As revealed by many recent studies, deficiency of NAD+ can be compensated by the NMN supplementation that affects a range of pharmacological activities in different disease conditions [13].
Several methods had been used to prepare and purify NMN:
incubation of diphosphopyridine nucleotide in a non-phosphate buffer and fluoride with potato pyrophosphatase, synthesis of NMN from nicotinamide by human hemolysates and erythrocytes, and specific hydrolysis of pyrophosphate bond of NAD+ using NAD+ pyrophosphatase and metal catalysts [7].
NMN을 제조하고 정제하기 위해 여러 방법이 사용되었습니다:
비-인산염 완충액 및 불소에서의 감자 피로포스파타제 인큐베이션,
인간 용혈액 및 적혈구에 의한 니코틴아미드로부터의 NMN 합성,
NAD+ 피로포스파타제 및 금속 촉매를 사용한, NAD+ 피로포스페이트 결합의 특정 가수분해[7].
그러나, 이들 방법은 그다지 효율적이지 않고, 적은 양의 NMN을 생성하여 NMN 가격이 높아집니다.
현재 미생물 생명공학이 NMN을 얻는 데 사용됩니다.
그럼에도 불구하고 NMN의 많은 비용과 순도 문제를 해결하려면 혁신적 방법과 최적화가 필수입니다.
NMN 생산비용을 효율적으로 만들기 위해, 박테리아와 효모를 사용하는 간단하고 효율적인 생명공학 생산 및 정제방법을 사용하는 많은 연구가 수행되고 있습니다[14].
However, these methods are not rather efficient and they produce low amounts of NMN, giving rise to high price of NMN.
Currently, microbial biotechnologies are used to obtain NMN.
Nevertheless, innovative methods and optimisations are essential in order to address the high cost and purity issues of NMN.
Many studies have been performed using simple and efficient biotechnological production and purification methods using bacteria and yeast to make NMN production cost effective [14].
처음에는 NMN이 NAD+ 생합성의 중간체이자 세포 에너지의 원천으로만 여겨졌으나, 현재는 NMN의 노화방지 활성과 다양한 건강상 이점 및 약리학적 활성에 대해 과학계 관심이 집중되고 있는데, 이는 NAD+ 복원과 관련이 있습니다.
Though at first, NMN was only considered as a source of cellular energy and an intermediate in NAD+ biosynthesis, currently, the attention of the scientific community has been paid on anti-aging activity and a variety of health benefits and pharmacological activities of NMN which are related to the restoring of NAD+.
따라서 NMN은, 연령 유발 2형 당뇨병(age-induced type 2 diabetes), 비만, 뇌 및 심장 허혈, 심부전 및 심근병증, 알츠하이머병 및 기타 신경변성 장애, 각막손상, 황반변성 및 망막변성, 급성 신장 부상 및 알코올성 간질환 등 다양한 질병에 대한 치료 효과가 있습니다[15].
Thus, NMN has therapeutic effects towards a range of diseases, including age-induced type 2 diabetes, obesity, cerebral and cardiac ischemia, heart failure and cardiomyopathies, Alzheimer’s disease and other neurodegenerative disorders, corneal injury, macular degeneration and retinal degeneration, acute kidney injury and alcoholic liver disease [15].
Mechanism underlying the anti-aging activity of NMN
NMN의 노화방지 활동의 기본 메커니즘
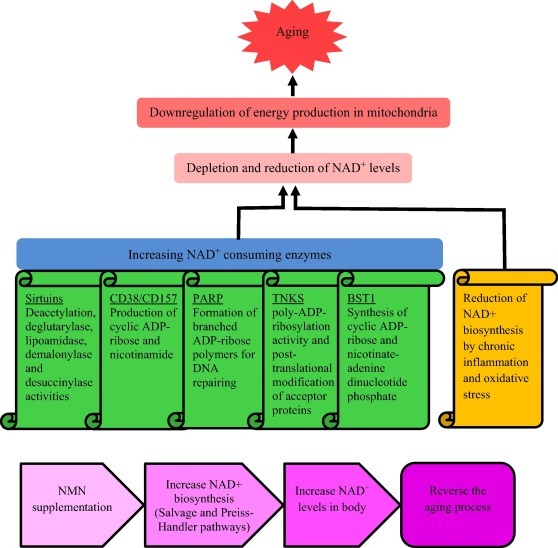
노화는 NAD+ 고갈로 인한 뇌, 지방조직, 피부, 간, 골격근, 췌장과 같은 다양한 기관의 미토콘드리아에서 에너지 생산의 하향조절에 의해 자연적 과정으로 식별됩니다[16].
Aging, as a natural process is identified by downregulation of energy production in mitochondria of various organs such as brain, adipose tissue, skin, liver, skeletal muscle and pancreas due to the depletion of NAD+ [16].
신체의 NAD+ 수치는 노화 시 NAD+ 소비효소 증가로 인해 감소합니다[17].
NAD+ levels in the body decrease as a consequence of increasing NAD+ consuming enzymes when aging [17].
이러한 NAD+ 소비효소에는 NADase(CD38/CD157), 폴리(ADP-리보스) 중합효소(PARP), NAD+ 의존성 아세틸라제(sirtuins), BST 및 탄키라제(TNKS)가 있습니다[18].
These NAD+ consuming enzymes include NADase (CD38/CD157), poly (ADP-ribose) polymerase (PARP), NAD+ dependent acetylase (sirtuins), BST and tankyrase (TNKS) [18].
Sirtuins consume NAD+ in order to execute a variety of functions such as deacetylation, deglutarylase, lipoamidase, demalonylase and desuccinylase activities.
Sirtuins는 deacetylation, deglutarylase, lipoamidase, demalonylase 및 desuccinylase 활성과 같은 다양한 기능을 실행하기 위해 NAD+를 소비합니다.
장수, 노화 및 연령 관련 생리학적 변화 조절은 시르투인 생물학의 실질적 측면 중 하나이며[19], CD38은 NAD+를 사용하여 순환 ADP-리보스 및 니코틴아미드를 생성합니다.
Regulation of longevity, aging and age-associated physiological changes is one of the substantial aspects of sirtuin biology [19], while CD38 utilises NAD+ to produce cyclic ADP-ribose and nicotinamide.
그 외에도 PARP는 NAD+를 사용하여 DNA 복구를 돕는 분지형 ADP-리보스 폴리머를 형성합니다[20].
NAD+를 소비하는 효소에 의한 이러한 고갈된 NAD+ 수준은 NMN이 NAD+ 생합성의 중간화합물이기 때문에 NMN을 신체에 투여함으로써 보상될 수 있습니다.
Apart from that, PARP expends NAD+ to form branched ADP-ribose polymers which assists in DNA repairing [20].
This depleted NAD+ level by NAD+ consuming enzymes can be compensated by administration of NMN to the body since NMN is an intermediate compound of the NAD+ biosynthesis.
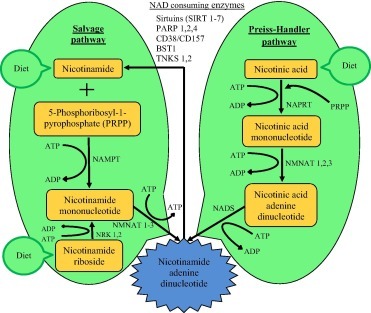
트립토판, 회수, Preiss-핸들러 경로로부터의 새로운 합성 등 포유류 세포에서 NAD+를 생성하는 3가지 다른 생합성 경로가 있습니다.
There are three different biosynthesis pathways to produce NAD+ in mammalian cells including de novo synthesis from tryptophan, salvage and Preiss-Handler pathways.
Among these three pathways, NMN is an intermediate by-product in salvage pathway, and it is involved in NAD+ biosynthesis through salvage and Preiss-Handler pathways as illustrated in Fig. 3 [15].
이 3가지 경로 중, NMN은 salvage pathway의 중간 부산물이며, salvage 및 Preiss-Handler 경로를 통한 NAD+ 생합성에 관여합니다(그림3)[15].
salvage 경로는 NAD+ 생합성의 가장 효율적이고 주요 경로로서, 니코틴아미드와 5-포스포리보실-1-피로포스페이트가 NAMPT의 효소 작용으로 NMN으로 전환된 후 ATP로 접합되고 NMNAT에 의해 NAD로 전환됩니다[21].
The salvage pathway is the most efficient and the main route for the NAD+ biosynthesis, in which nicotinamide and 5-phosphoribosyl-1-pyrophosphate are converted to NMN with the enzyme action of NAMPT followed by conjugation to ATP and conversion to NAD by NMNAT [21].
또한 NAD+를 소비하는 효소는 NAD+ 분해와 결과적으로 부산물로 니코틴아미드를 형성하는 역할을 합니다[13].
Furthermore, NAD+ consuming enzymes are responsible for degradation of NAD+ and consequent formation of nicotinamide as a by-product [13].
Preiss-Handler 경로에서, 처음에는 니코틴산이 NAPRT(nicotinic acid phosphoribosyl-transferase enzyme) 활성에 의해 니코틴산 모노뉴클레오타이드(NAMN)로 전환되고, 이어서 니코틴아미드/니코틴산 모노뉴클레오티드 아데닐릴트랜스퍼라제(NMNAT 1/2/3)를 사용한, NAMN에서 니코틴산 아데닌 디뉴클레오티드(NAAD+)가 생합성됩니다.
In the Preiss-Handler pathway, initially, nicotinic acid is converted to nicotinic acid mononucleotide (NAMN) by nicotinic acid phosphoribosyl-transferase enzyme (NAPRT) activity followed by biosynthesis of nicotinic acid adenine dinucleotide (NAAD+) from NAMN using nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT 1/2/3).
그 후, NAAD+는 ATP와 암모니아를 사용하는 NAD+ 합성효소(NADS)에 의해 NAD+로 변환됩니다[22].
Subsequently, NAAD+ is transformed to NAD+ by NAD+ synthetase (NADS) using ATP and ammonia [22].
Download : Download high-res image (319KB)Download : Download full-size image
Fig. 3. NAD+ biosynthesis pathways in which NMN involves. Biosynthetic pathways of NAD+ in mammalian cells in which NMN involves are Preiss–Handler and salvage pathways, and the salvage pathway is the major source of NAD+. NAMPT-nicotinamide phosphoribosyltransferase; ATP-adenosine triphosphate; ADP-adenosine diphosphate; NMNAT-nicotinamide mononucleotide adenylyltransferase; NRK-nicotinamide riboside kinases; NAPRT-nicotinate phosphoribosyltransferase; NADS-nicotinamide adenine dinucleotide synthetases.
노화와 함께 발생하는 만성염증과 산화스트레스는 NAMPT 매개 NAD+ 생합성의 감소 및 억제의 원인입니다[23].
Chronic inflammation and oxidative stress, which come along with aging, are the causes for reduction and inhibition of NAMPT-mediated NAD+ biosynthesis [23].
특히 핵 기원인 노화와 함께 NAD+ 함량의 고갈은 PCG-1α/β-독립적 산화-인산화 경로의 미토콘드리아 조절중단과 관련있으며, 이는 가성 저산소증을 유발합니다.
이 사건은 NAD+ 함량을 높이면 뒤집힐 수 있습니다[24].
The depletion of NAD+ contents along with aging, which particularly of nuclear origin, is associated with interruption of mitochondrial regulation of PCG-1α/β-independent pathway of oxidative-phosphorylation as well, causing pseudohypoxia.
This incident can be overturned by raising the NAD+ content [24].
미토콘드리아 기능을 감소시키는 것 외에도 인지장애, DNA 손상 및 시르툴린 유전자 비활성화와 같은 생물학적 변화는 신체의 NAD+ 수를 증가시켜 피할 수 있는 노화로 인해 발생합니다[24].
Apart from reducing the functions of mitochondria, biological changes such as cognitive impairments, DNA damage and sirtulin gene inactivation, are brought about by aging which can be evaded by enhancing NAD+ count in the body [24].
NMN 보충 외에도 신체의 NAD+ 수치는 낮은 에너지 섭취[25], 칼로리 제한, 단식, 신체의 포도당 함량부족 및 운동과 관련된 상태에 대한 반응으로 증가할 수 있습니다.
그럼에도 불구하고 NAD+ 수치는 고지방 식이 섭취와 노화결과로 감소합니다[26].
Apart from NMN supplementation, NAD+ levels in the body can be increased as a response to conditions related to lower energy intake [25], calorie restriction, fasting, lack of glucose content in the body and exercise.
Nevertheless, NAD+ levels decrease as a consequence of intake of high-fat diets and aging [26].
현재 여러 연구에서 NAMPT 반응 생성물인 NMN이 SIRT1 활성을 유발하는 데 활용될 수 있음을 보여주었습니다[27].
Currently, several studies have shown that NMN, the NAMPT reaction product, is able to be utilised to trigger the SIRT1 activity [27].
NAD+ 수치가 충분하지 않으면 SIRT1은 저산소증 유발인자 1 알파(HIF-1)를 차단할 수 없게 되며, 수치가 높아지면 세포 수준에서 미토콘드리아와 핵 사이의 통신 그리고 전신 수준에서 지방조직과 시상하부 사이의 통신이 차단됩니다[28].
It has been shown that when there is not adequate NAD+ levels, SIRT1 becomes unable to block hypoxia-inducible factor 1 alpha (HIF-1) and elevated levels of which blocks the communication between mitochondria and nucleus at the cellular level and between adipose tissue and hypothalamus at the systemic level [28].
결과적으로 미토콘드리아와 핵 커뮤니케이션이 중단되면, 미토콘드리아 기능이 급격히 감소하여 노화관련 합병증과 질병이 발생합니다.
The resulting interruption in mitochondria and nuclear communication causes swift reduction in mitochondrial function that leads to the development of age-associated complications and diseases.
Nevertheless, the particular communication and mitochondrial function can be restored by the administration of NMN as the NAD+ precursor [24].
그럼에도 불구하고 NMN을 NAD+ 전구체로 투여하면 특정 통신 및 미토콘드리아 기능을 회복할 수 있습니다[24].
노화 시 NAD+ 수치를 감소시키는 원인과 NMN의 노화방지 활동의 기본 메커니즘이 그림4에 설명되어 있습니다.
Causes for reducing NAD+ levels when aging and mechanism underlying anti-aging activity of NMN are illustrated in Fig. 4.
기능식품산업은 NAD+ 수치를 향상시키기 위해 이미 NMN을 매우 효율적이고 실행 가능한 노화방지 건강제품으로 적극적으로 마케팅하기 시작했으며[15], 따라서 일반인에게 장수를 제공합니다.
The nutraceutical industry has already started to market NMN aggressively as a highly efficient and viable anti-aging health product to enhance the NAD+ levels [15], thus to provide longevity to the general population.
추가로, NMN의 잠재적 노화방지 활성과 그 적용 가능성 및 사용성을 조사하기 위한 많은 연구가 수행됩니다.
In addition, many studies are carried out to investigate the potential anti-aging activity of NMN and their applicability and usability.
Download : Download high-res image (552KB)Download : Download full-size image
Fig. 4. Causes for reducing NAD+ levels when aging and mechanism underlying anti-aging activity of NMN. DNA damage, chronic inflammation, oxidative stress and increasing NAD+ consuming enzymes (sirtuins, CD38/CD157, PARP, TNKS and BST) accelerate NAD degradation. The reduced levels of NAD+ cause downregulation of energy production in mitochondria, leading to aging and various age-associated diseases. NMN supplementation can reinstate NAD+ levels in the body through biosynthesis pathways, reversing the aging process and preventing age-associated diseases.
Promises and efficacy as an anti-aging health product
안티에이징 건강제품으로서의 약속과 효능
세포배양, 동물모델 및 인간 임상조사를 사용하여 노화 및 노화관련 합병증 및 질병을 관리하고 조절하기 위한 노화방지 건강제품으로서 NMN의 약속과 효능을 조사하기 위해 많은 연구가 수행되었습니다.
Many studies have been carried out to investigate the promises and efficacy of NMN as an anti-aging health product for managing and regulating aging and age-associated complications and diseases using cell culture, animal models and human clinical investigations.
In vivo studies, which have been carried out to investigate anti-aging therapeutic effects of NMN administration, are summarised in Table 1, including animal models, given NMN dose, duration and observed effects.
NMN 투여의 항노화 치료효과를 조사하기 위해 수행된 생체 내 연구는 NMN 투여량, 기간 및 관찰된 효과가 주어진 동물모델을 포함하여 표1에 요약되어 있습니다.
Yoshino 등에 따르면[23], 노화과정에서 다양한 기관에서 NAMPT 및 NAD+ 수준이 현저히 감소하고 NMN 투여는 노화 유도형에서 NAD+ 수준 강화(500에서 1550 pmol/mg 조직), 인슐린 분비, 인슐린 감수성 및 당뇨병 쥐 2마리의 지질 프로필을 향상시킬 수 있습니다.
According to Yoshino et al. [23], during the aging process, NAMPT and NAD+ levels significantly decrease in various organs and NMN administration could enhance NAD+ levels (from 500 to 1550 pmol/mg-tissue), insulin secretion, insulin sensitivity and lipid profile in age-induced type 2 diabetic mice.
NMN의 투여는 또한 일주기 리듬, 염증반응 및 산화스트레스와 관련된 유전자 발현을 회복시키고, 부분적으로 SIRT1 활성화에 의해 간 인슐린 감수성을 개선할 수 있습니다.
Administration of NMN also can restore gene expression linked to circadian rhythm, inflammatory response and oxidative stress, and improve hepatic insulin sensitivity, partially by SIRT1 activation.
Table 1. Anti-aging therapeutic effects of NMN administration in vivo.
|
Therapeutic effect/age-related complication
|
Model
|
NMN dose and duration
|
Effect
|
Reference
|
|
Age- and diet-induced diabetes
|
Age-induced and high-fat-induced diabetic mice
|
IP − 500 mg/kg body weight/day, age induced mice – 11 days, high-fat induced mice – 7–10 days
|
Enhanced insulin secretion, insulin sensitivity and lipid profile in age-induced type 2 diabetic mice
Improved hepatic insulin sensitivity and glucose tolerance in high-fat-induced diabetic mice
|
|
|
Age-associated vascular dysfunction and oxidative stress
|
Aged (26–28 months) C57BI/6 mice
|
Drinking water − 300 mg/kg body weight/day, 8 weeks
|
Restored maximum carotid artery endothelium‐dependent dilation and nitric oxide‐mediated carotid artery endothelium‐dependent dilation
Reduced vascular oxidative stress, normalised aortic stiffness and activated vascular SIRT1 activity
|
|
|
Anti-aging activity and longer retention in the body
|
Wistar rats (7 weeks)
|
IP − 45 µmol/kg body weight, Single
|
Retained in the body for longer period than nicotinamide Resulted a higher yield of NAD+ activating higher response of SIRT1 than nicotinamide and better anti-aging activity and longevity than nicotinamide
|
|
|
Alzheimer's disease
|
APP(swe)/PS1(ΔE9) double transgenic (AD-Tg) mice
|
Subcutaneously − 100 mg/kg body weight/every other day, 28 days
|
Enhanced mitochondrial bioenergetic functions
Reduced expression of amyloid precursor protein (APP)
|
|
|
Age-associated physiological decline
|
Wild-type C57BL/6N mice
|
Drinking water − 100 and 300 mg/kg body weight/day, 12 months
|
Suppressed aging-induced body weight gain
Ameliorated eye functions, healthy plasma lipid profile, insulin sensitivity, physical activity, energy metabolism and other physiopathologies
Averted alterations in age-associated gene expression Enhanced mitonuclear protein imbalance and mitochondrial oxidative metabolism in skeletal muscles
|
|
Therapeutic effect/age-related complication
|
Model
|
NMN dose and duration
|
Effect
|
Reference
|
|
Alzheimer's disease
|
Intracerebroventricular infusion of Aβ1-42 oligomer in Wistar rats
|
IP − 500 mg/kg body weight/day, 10 days
|
Restored learning and cognition
Enhanced energy metabolism and neuron survival Eliminated ROS accumulation
|
|
|
Age-associated susceptibility to acute kidney injury
|
Aged (20 months) wild-type 129S2/Sv and C57BL/6 mice
|
IP − 500 mg/kg body weight/day, 4 days
|
Boosted NAD+ and SIRT1 levels in aged kidneys
Protected aged kidneys from both ischemia–reperfusion- and cisplatin-induced acute kidney injuries
|
|
|
Age-associated decline in DNA repair capacity
|
Aged (20–26 months) C57BL/6J mice
|
IP − 500 mg/kg body weight/day, 7 days
|
Reduced DND damage
Protected against changes in haemoglobin and white blood cell count including lymphocytes
|
|
|
Alzheimer's disease
|
APP(swe)/PS1(ΔE9) double transgenic (AD-Tg) mice
|
Subcutaneously − 100 mg/kg body weight/every other day, 28 days
|
Improved of behavioural measures of cognitive impairments
Reduced inflammatory responses, synaptic loss, amyloid plaque burden and β-amyloid production by inhibition of JNK activation
|
|
|
Age-related vascular aging
|
Aged (18 months) C57BL/6J mice
|
Drinking water − 400 mg/kg body weight/day, 2 months
|
Increased endurance
Improved blood flow in elderly mice by increasing capillary density
|
|
|
Age-related cognitive and behavioural dysfunction
|
Aged (20 months) C57BL/6 mice
|
Per oral − 300 mg/kg body weight/day, 3 weeks
|
Mitigated age-related decline in the sensory processing of hypersensitivity, some aversive stimuli and other related behaviours
|
|
|
Aging-induced cognitive impairment
|
Aged (24 months) Wistar rats
|
IP − 100 mg/kg body weight/every other day, 28 days
|
Stimulated neuroprotective effects
Reduced aging-induced cognitive decline
Alleviated age-associated memory and learning impairments
|
|
Therapeutic effect/age-related complication
|
Model
|
NMN dose and duration
|
Effect
|
Reference
|
|
Promoting micro-RNA profile in the aorta, epigenetic rejuvenation and anti-atherogenic effects
|
Aged (24 months) C57BL/6 mice
|
IP − 500 mg/kg body weight/day, 14 days
|
Overturned age-associated transformations in micro-RNA profile in the aged mouse aorta
|
|
|
Alzheimer's disease
|
C57BL/6 mice and transgenic mice with mitochondria‐targeted yellow fluorescence protein (mito‐eYFP) expression in neurons
|
IP − 62.5 mg/kg, Single
|
Enhanced mitochondrial bioenergetics
Overturned physiological decline
Restrained postischemic NAD+ depletion and cellular death
Inhibited mitochondrial excessive fragmentation
Reduced mitochondrial protein acetylation and ROS in the hippocampus
|
|
|
Skeletal aging
|
Aged (12 months) C57BL/6J mice
|
Drinking water − 300 mg/kg body weight/day, 3 months
|
Protected from skeletal aging
Reduced osteogenesis
Increased adipogenesis by regulating mesenchymal stromal cells
|
|
|
Age-associated cerebromicrovascular dysfunction and cognitive decline
|
Aged (24 months) C57BL/6 mice
|
IP − 500 mg/kg body weight/day, 14 days
|
Restored NAD+ levels mitochondrial energetic
Reduce oxidative stress
Reversed cerebrovascular endothelial dysfunction
Improved neurovascular coupling responses and cognitive performance
|
|
|
Rescuing female fertility during reproductive aging
|
Aged (12–14 months) C57BL/6 female mice
|
Drinking water − 2 g/L, 4 weeks
|
Restored NAD+ levels and fertility
Rejuvenated oocyte quality
Supported the embryo development reversing the adverse consequences of elevated maternal age
|
|
Therapeutic effect/age-related complication
|
Model
|
NMN dose and duration
|
Effect
|
Reference
|
|
Mitochondrial function and cardioprotection in myocardial ischemia/reperfusion injury
|
Aged Wistar rats (22–24 months old)
|
IP − 100 mg/kg body weight/every other day, 28 days
|
Enhanced hemodynamic parameters
Decreased dehydrogenase release and infarct size
Ameliorated mitochondrial membrane potential
Declined mitochondrial ROS and oxidative stress
Restored NAD+/NADH ratio
|
|
|
Promoting neurovascular rejuvenation
|
Aged (24 months) C57BL/6 mice
|
IP − 500 mg/kg body weight/day, 14 days
|
Promoted SIRT1activatio in the neurovascular unit
Reversed age-associated alterations in neurovascular transcriptome which predicted to be mediated by the involvement of genes in anti-apoptosis, anti-inflammatory and mitochondrial rejuvenation pathways
|
|
|
Oocyte quality reduction with advanced maternal age
|
Aged (64–68 weeks) ICR female mice
|
IP − 200 mg/kg body weight/day, 10 days
|
Restored NAD+ levels
Increased ovulation, fertilisation capability and meiotic competency
Promoted cytoplasmic and nuclear maturation
Suppressed accumulation of DNA damage and ROS
Declined apoptosis
|
|
|
Werner syndrome
|
young
(Day 2) and old (Day 10) wrn-1(gk99) and wild type N2 Caenorhabditis elegans and Drosophila melanogaster worms
|
1 mM from the L4 stage
|
Extended lifespan and health-span
Improved proliferative potency and number of mitotic cells by the NAD+ repletion
|
|
|
Ataxia telangiectasia
|
Ataxia-telangiectasia mutated B6;129S4-Atmtm1Bal/J mice
|
Drinking water − 12 mM, 2 weeks
|
Normalized neuromuscular function
Delayed memory loss
Extended lifespan
Stimulated neuronal DNA repair
Improve mitochondrial quality via mitophagy
|
IP-Intraperitoneal.
De Picciotto et al. [29] found that NMN supplementation was capable of restoring NAD+ levels (by threefold), vascular SIRT1 activity, maximum carotid artery endothelium‐dependent dilation, and nitric oxide‐mediated carotid artery endothelium‐dependent dilation in mice. Kawamura et al. [30] have reported that NMN retained in animals for longer period than nicotinamide. NMN resulted in a higher yield of NAD+ (80 nmol/g of liver tissue) in salvage biosynthesis pathway activating higher response of SIRT1 than nicotinamide.
Mills et al. [10] found that devoid of any apparent deleterious effect or toxicity, NMN effectively suppressed aging-induced body weight gain and ameliorated eye dysfunction in mice. It maintained healthy plasma lipid profile, insulin sensitivity, physical activity, energy metabolism and other physiopathologies. Additionally, NMN supplementation averted alterations in age-associated gene expression in main metabolic organs, while enhancing mito-nuclear protein imbalance and mitochondrial oxidative metabolism in skeletal muscles. Guan et al. [31] elaborated that NMN supplementation restored reduced contents of renal protective molecule, SIRT1 and its cofactor, NAD+. The heightened NAD+ and SIRT1 levels in kidneys of aged mice protected aged kidneys from both ischemia–reperfusion- and cisplatin-induced acute kidney injuries.
As explained by Li et al. [32], nudix homology domains (NHDs) are binding domains of NAD+ and through binding to them, NAD+ is able to regulate protein–protein interactions. The modulation of these interactions may lead to protecting the human body from aging, radiation and cancer. PARP1 is a critical protein that involves in DNA repairing. The inhibition of PARP1 is prevented by binding of NAD+ to the NDH domain of DBC1 (deleted in breast cancer 1) nuclear protein. Nevertheless, when NAD+ concentration is reduced with the age, DBC1 is progressively bound to PARP1, leading to accumulate DNA damage. This process of DNA damage can be reversed by restoring NAD+ levels in the body. Results of this in vivo study showed that, NMN treatment increased hepatic NAD+ contents, disrupted DBC1-PARP1 complex, reduced DNA damage and defended against changes in haemoglobin and white blood cell count including lymphocytes.
Tsubota [33] showed that NMN, as a sirtuins activating agent had protective effects against age-related ocular diseases such as glaucoma, dry eye and macular degeneration. As elaborated by Das et al. [34], reduction of blood flow and capillary density with aging is a main cause of morbidity and mortality, whereas NMN as a NAD+ precursor can reverse these to a certain extent by triggering sirtuin deacylases (SIRT1-7). NMN supplementation could increase NAD levels in liver and gastrocnemius tissues by nearly 5 and 1 folds, respectively. Johnson et al. [35] observed that in vivo occurrence of age-associated cognitive and behavioural dysfunctions was induced by declined NAMPT-mediated NAD+ biosynthesis causing 40% gradual decrease of NAD+ levels in the hippocampus, predominately in CA1 region. It showed that, even by short term supplementation of NMN, NAD+ levels could be restored, while mitigating the age-related changes in the sensory processing of hypersensitivity, several other aversive stimuli and other associated behaviours, enhancing the quality of later lives. A prospective downstream effector was identified, namely, calcium/calmodulin-dependent serine protein kinase, which got reduced in hippocampus along with age-related NAD+ drop. The promoter activity of this effector was regulated in a SIRT1 reliant manner, while its expression could be enhanced by NMN supplementation.
Hosseini et al. [36] reported that in vivo intraperitoneal injection of NMN and NMN together with melatonin stimulated neuroprotective effects and alleviated age-associated memory and learning impairments. Furthermore, the administration of them separately or in combination enhanced mitochondrial function and decreased apoptosis cell count both in hippocampus and prefrontal cortex regions of aged rats. Kiss et al. [37] illustrated that age-associated NAD+ decline was linked with mis-regulation of vascular mico-RNA expression, NMN intraperitoneal treatments resulted in anti-aging transformations mouse aorta mico-RNA expression profile. It was predicted that epigenetic rejuvenation and anti-atherogenic effects were some of regulatory consequences of NMN. NMN treatment distinctively expressed mico-RNAs in aged vessels.
The role of NMN in fighting against age-associated disorders, such as skeletal aging associated with reduced osteogenesis and increased adipogenesis by regulating mesenchymal stromal cells (MSCs), was studied by Song et al. [38]. MSCs are non-hematopoietic stem cells that contain regeneration capacity. NMN supplementation led to self-renewal of MSCs along with decreased adipogenesis and strengthened osteogenesis through upregulating SIRT1 activity in mice. In addition, NMN has been identified as a promising and potential therapeutic agent for skeletal aging which is able to regulate bone-fat imbalance via SIRT1 and rejuvenation and expansion of aged MSCs.
Tarantini et al. [39] found that age-related increase of oxidative stress and cerebromicrovascular dysfunction, which exacerbated neurovascular coupling responses and age-related cognitive decline, were caused by reduced NAD+ availability with aging. NMN supplementation could restore tissue NAD levels by fold change of 1 and overturn these processes which led to improved cognitive performance in aged mice. Hosseini et al. [40] examined the individual and the combined outcome of NMN preconditioning and melatonin postconditioning on mitochondrial function and cardioprotection in myocardial ischemia/reperfusion injury of aged Wistar rats, because ischemic heart diseases are the foremost reasons for mortality and disability in elderly. This treatment ameliorated mitochondrial membrane potential, declined mitochondrial ROS and oxidative stress and restored the balance between the oxidised and reduced forms of NAD. The consequences of the combined therapy of NMN and melatonin on these beneficial effects were greater than those of individual treatments.
According to Kiss et al. [41] in vivo NMN supplementation enhanced NAD+ levels followed by promoting SIRT1 activation and improving neurovascular functions and cognitive performances. These protective effects caused by NMN treatments on neurovascular function were predicted to be mediated by the involvement of genes in anti-apoptosis, anti-inflammatory and mitochondrial rejuvenation pathways which are attributable to versatile sirtuin-regulated anti-aging alterations in the neurovascular gene expression.
Miao et al. [42] showed that NMN supplementation improved the oocyte quality through restoring NAD+ levels (nearly 50%) in mice. It increased ovulation, fertilisation capability and meiotic competency, while promoting cytoplasmic and nuclear maturation in order to maintain euploidy. Furthermore, NMN reinstated mitochondrial functions of aged oocytes to mitigate accumulation of DNA damage and reactive oxygen species, leading to low levels of apoptosis. This finding is echoed by Bertoldo et al. [43] who further indicated that restoration of fertility in aged mice and other benefits of NMN treatment could be recapitulated by the transgenic overexpression of SIRT2. Apart from rejuvenating oocyte quality of aged animals, NMN supported the embryo development, reversing the adverse consequences of elevated maternal age on developmental milestones by increasing NAD levels in ovarian tissue from nearly 200 to 300 pmol/mg. Fu and Zhang [44] conducted an experiment for a patent application for using β-NMN in preparation of anti-aging health-care products or drugs using aged mice. It was found that the NMN administration could extend the life span of mice by ~29%. Aging is the most influential determinant and the greatest known risk factor for neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD) [45]. It has been illustrated that elevation of NAD+, sirtuins, CD38, PARP and SARM1 (sterile alpha and TIR motif containing 1) protein levels through NMN supplementation were capable of inducing neuronal mitophagy to ameliorate cognitive decline in Caenorhabditis elegans model of AD [46].
Long et al. [47] suggested that depletion of cellular and mitochondrial NAD+ contents induced mitochondrial abnormalities, activation of NAD glycohydrolases and bioenergetic failure as well as cell death. NMN supplementation decreased mitochondrial fragmentation and mutant amyloid precursor protein levels (38%) in mice without observing any detrimental side effects, while it enhanced mitochondrial bioenergetic functions.
Wang et al. [48] indicated that intraperitoneal administration of NMN restored learning and cognition in AD model Wistar rats, while enhancing energy metabolism and neuron survival and preventing ROS accumulation and the central biomarker (amyloid beta) induced neuronal death. Yao et al. [49] has also reported improvements of behavioural measures of cognitive impairments and reduction of multiple AD-linked pathological characteristics such as inflammatory responses, synaptic loss, amyloid plaque burden and β-amyloid production by inhibition of JNK activation in mice after subcutaneous administration of NMN.
According to Klimova et al. [50], in vivo NMN administration enhanced mitochondrial bioenergetics, overturned physiological decline and restrained postischemic NAD+ depletion and cellular death. Furthermore, increasing NAD+ levels in mitochondria (from 3.12 to 5.51 nmol/mg) by NMN supplementation caused many metabolic changes such as SIRT3-mediated decline in mitochondrial protein acetylation, which defended mitochondria by detrimental effects of ROS and excessive and impetuous fragmentation. In addition, according to Lu et al. [51], NMN could cause considerable beneficial effects, attenuate apoptosis and enhance mitochondrial inhibitor‑induced declining of energy metabolism in PD-like neuropathological and behavioural changes as resulted in cellular model of PD, utilising rotenone-treated PC12 cells. They investigated increased ATP1 and NAD+ levels (30%) in PC12 cells, necrotic and apoptotic cell death and cell survival with NMN supplementation.
Fang et al. [52] investigated that approximately two folds repletion of NAD+ through NMN unusually delayed accelerated aging and extended lifespan of Caenorhabditis elegans and Drosophila melanogaster models of Werner syndrome. A short-term treatment of NAD+ precursor, nicotinamide riboside (NR) improved cochlear health, restored outer hair cell loss and prevented hearing loss in CSBm/m mice [53]. It showed that, as a precursor of NAD+, NMN may have the same potential to prevent the age-related and Cockayne syndrome-related hearing loss. Fang et al. [54] demonstrated that boosting NAD+ through NMN supplementation evidently extended in vivo lifespan, normalized neuromuscular functions, stimulated neuronal DNA repair and ameliorated neuropathological defects.
Safety concerns and challenges
The ultimate goal of geroscience is to discover approaches to boost natural defence mechanisms and prolong healthy lifespan through better management of the risks posed to cells and tissues of humans. Due to the continuous enhancement of the living standards of people, the desire of healthy longevity has become progressively stronger. Conversely, there is a possibility to achieve the maximum life span specified by the nature through decelerating the rate of aging. Using tiny molecules to slow down the aging process and enhancing aging-associated outcomes is a flourishing research area at the present [55]. Furthermore, at the present, the number of anti-aging medicines and health products are commercially available and popular among elderly consumers [56]. Along with the current concerns on aging as a natural biological process, many researches on longevity are being conducted to understand and manage the aging process through anti-aging interventions, while complying with gerontological and biogerontological aspects.
Fu and Zhang [44] have applied for a patent application for using β-NMN in the preparation of healthcare products or anti-aging drugs as a single dose of 1–500 mg/kg body weight/day of β-NMN. The healthcare products or anti-aging drugs was in the forms of injections, enteric-coated preparations, aqueous solutions, granules, capsules or tablets. A number of NMN anti-aging health products have been produced and launched by various pharmaceutical, nutraceutical, biotechnology and health food companies. The quantity of NMN in available commercial products vary from 50 to 150 mg/capsule, whereas some consumers take two 150 mg capsules per day [57]. Nowadays, there are NMN products, marketed as supplements for anti-aging and longevity in the form of capsules, which are very high in dose such as ≥500 mg. The safety of these doses cannot be assessed since required clinical and toxicological studies have not been completed yet to establish the recommended safe levels for long term administration. Nevertheless, their safety and efficacy are uncertain and unreliable since most of them have not been backed up by rigorous scientific preclinical and clinical testing. This issue has been arisen as manufacturers are hesitant to pay for research and clinical trials due to potential lower profit margin, and there is no authorising agency to regulate NMN products because it is often sold as functional food product rather than heavily regulated therapeutic drug. Therefore, more strict approval process has been demanded by consumer advocacy groups requesting regulatory agencies to set standard and restrictions for marketing anti-aging health products, considering safety, health and wellbeing of consumers [58]. NMN should not be considered as a panacea for the elderly, because boosting NAD levels when not required may yield some detrimental effects. Therefore, the dose and frequency of NMN supplementation should be carefully prescribed depending on the type of age-related deficiency and all other confronting health conditions of the people [59]. Other NAD precursors have been studied to discover the efficacy for diverse age-related deficiencies and they are used for particular deficiencies, only after they are proven for effectiveness and safe to use. Therefore, the same principle should be applied to NMN as well [60], [61].
Grozio et al. [62] reported that NMN was speedily absorbed in the small intestine by a specific transporter, which was encoded by the Slc12a8 gene as demonstrated in in vitro and in vivo studies. Even though a large number of preclinical studies have provided a highly promising possibility for developing NMN as an evidence-based anti-aging health product, its safety and effectiveness on human physiology remains unclear. The toxicological and clinical evidence to back up its utility is currently scarce. Despite this, NMN supplements are already available in the market and considerably used by consumers as anti-aging health products. Therefore, in addition to animal models, human trails to investigate the safety and efficacy of NMN should be conducted focusing on toxicological parameters and safe metabolite levels in the human body [8].
Increased attention on potential pro-longevity effects of NAD+ precursors over the recent years has led to increased consumption of nicotinamide either as a clinical treatment or a dietary supplement, raising concerns on the safety of their long-term usage. Nicotinamide has been found to cause adverse effects on several organs in the human body such as kidney, liver, pancreatic β-cells and cells in plasma and induce nausea, stomach discomfort and headaches [63], [64]. In addition, high dose of nicotinamide supplementation is associated with decreased insulin sensitivity and increased oxidative stress [64]. However, NMN supplementation has been found to have significant recovering effects on hepatocyte functions and liver pathologies in early-stage of ethanol toxicity, instead of causing adverse effects to the liver [65]. It has also been found to improve insulin sensitivity and oxidative stress in animal models [23], [29].
Although, nicotinamide supplementation induced DNA damage and oxidative DNA damages in various tissues of the body such as renal and hepatic tissues [66], NMN has been proven to reduce DNA damage and accumulation of ROS [32], [42]. Supplementation of nicotinamide caused neurodegeneration of dopaminergic neurons and structural brain changes and behavioural deficits in rats [67]. Supplementation of NMN has been demonstrated to stimulate neuroprotective effects and ameliorate cognitive decline and behavioural dysfunctions [35], [36]. Rolfe [68] reported that administration of nicotinamide and niacin may cause development of PD, whereas supplementation of NMN has been found to be a promising therapeutic remedy for PD [51].
NR is also a precursor of NAD+ and administration of NR has the capability to elevate body NAD+ levels. According to the results of clinical trials that have been performed to assess the safety of NR administration, NR supplementation increased LDL cholesterol levels in the body [69] and enhanced fatty liver conditions [70] as adverse effects. Nevertheless, NMN administration was observed to improve plasma lipid profile, ameliorating free fatty acids, triglycerides and cholesterol levels and lower intrahepatic triglyceride levels in mice [10]. Shi et al. [71] have shown that excessive dose of NR generated white adipose tissue dysfunction and heightened insulin resistance in mice. However, contradictory results have been reported with NMN supplementation as it reduced adiposity and enhanced insulin sensitivity [24], [72].
NMN as another precursor of NAD+, has the potential to encounter similar safety concerns and challenges as other NAD+ precursors. Thus, further clinical studies on the safety and toxicology of NMN are urgently needed. Mehmel et al. [60] argued that NR is a more suitable NAD+ precursor as an anti-aging treatment. This highlights the needs to study adverse effects of nicotinamide and NMN, even though NMN appears to have considerable beneficial pharmacological actions in preclinical studies. Yoshino et al. [57] conducted a clinical trial, supplementing 250 mg/day of NMN for 10 weeks to 25 prediabetes women who were overweight or obese in order to identify effect on body composition, gene expression profile, insulin signalling and insulin sensitivity and observed potential metabolic benefits without any adverse effects.
A toxicological study for NR chloride has been done in a clinical setting, and the no observed adverse effect level (NOAEL) was 300 mg/kg/day and the lowest observed adverse effect level was 1000 mg/kg/day [73]. No such data is available for human administration of NMN yet. However, Cros et al. [74] investigated subchronic oral toxicity, acute oral toxicity, genotoxicity and mutagenicity of high purity synthetic form of NMN (NMN-C®) using female Sprague-Dawley rats (7 weeks old). According to the results, NOAEL of NMN-C® was ≥1500 mg/kg/d. At an oral administration dose of 2666 mg/kg of NMN-C®, no treatment- associated adverse effects or mortality were nor resulted. NMN-C® did not show toxic effects and appeared to be safe over a 90-day sub-chronic period of repeated oral administration at doses of 375, 750 and 1500 mg/kg/d.
Conze, Crespo-Barreto, and Kruger [75] determined safety of a synthetic form of NR namely, NiagenTM using in vitro and rat toxicological study, and found that it was not genotoxic. The lowest observed adverse effect level for NiagenTM was 1000 mg/kg body weight/day and NOAEL was 300 mg/kg body weight/day. Furthermore, NR has been examined in six clinical trials, where it has been established that it is safe for short-term (8 days) and long-term (6 weeks) supplementation in compliance with confirmed oral availability [60]. These values have not been established regarding NMN supplementation. Furthermore, recently, NR has been granted Generally Recognised as Safe (GRAS) status by the US Food and Drug Administration (FDA), which is yet to be achieved for NMN.
Mills et al. [10] reported that in vivo long-term administration of NMN mitigated age-associated physiological decline, effectively without generating noticeable toxicity, deleterious side effects or raised mortality. Tsubota [76] reported that the first phase I human clinical study (UMIN000021309) has been initiated aiming at evaluating the bioavailability, mechanism of action and the safety of NMN in the human body. This has been a collaborative study between Keio University School of Medicine in Tokyo and Washington University School of Medicine in St. Louis. Using ten healthy volunteers, the time course of blood NMN concentration and the safety of NMN administration were assessed, which was intended for developing anti-aging nutraceutical. However, the research team has been unsuccessful in detecting NMN in plasma samples.
Irie et al. [77] conducted a non-blinded clinical trial using 10 healthy men to investigate the safety of oral NMN administration and the pharmacokinetics of nicotinamide metabolites at the Keio University School Medicine, Japan. They found that single oral administration of 100, 250 and 500 mg of NMN doses was well-tolerated and safe since it did not cause any observable clinical symptoms or changes in body temperature, oxygen saturation, blood pressure and heart rate. In addition, significant changes in ophthalmic, ocular fundus and neurological system parameters were not observed after NMN administration. There were no changes in the results of laboratory analysis of urine and blood as well as sleep quality and score before and after the NMN administration. Oral administration of NMN increased serum bilirubin contents and decreased blood glucose, chloride and serum creatinine levels, but within the normal range. NMN administration did not increase nicotinamide in blood to the level which causes adverse effects associated with high dose of nicotinamide. Since the safety of single oral administration of NMN has only been considered in this study, further clinical investigations are essential to be performed to assess the safety and efficacy of long-term administration of NMN. The organs and the tissue NMN levels have not been analysed in this study, which should be considered in future clinical studies.
As stated by Hong et al. [15], three more human clinical studies are being carried out to evaluate the safety concerns of NMN administration. One study is a phase II study (UMIN000030609), which has been initiated by the Keio University School of Medicine, Japan to evaluate the safety of long-term administration of NMN, pharmacokinetics and metabolites of NMN, and its effect on glucose metabolism in healthy adults. Another study is being performed to assess the effect of long-term intake of NMN on different hormones in healthy individuals (UMIN000025739) by the Hiroshima University, Institute of Biomedical and Health Sciences. The third clinical study (UMIN000036321) has been designed to assess the consequences of oral administration of NMN on the body composition in elderly people at the University of Tokyo Hospital. These clinical trials are still ongoing and there is no result published yet. Yoshino et al. [23] have also highlighted the importance of more comprehensively assessing potential adverse effects of NMN by conducting preclinical and clinical studies, considering different dietary conditions as well.
According to Di Stefano et al. [78], inhibitors of NMN synthesising enzyme (NAMPT), provided robust functional and morphological protection of injured synapses and axons, regardless of reducing NAD. But, exogenous NMN eliminated this protection with the accumulation of NMN after nerve injury and NMNAT2 degradation advanced axon degeneration. They suggested that the relationship between the increase of NMN and axon degeneration could be a long-hypothesised toxic and deleterious factor. Poljsak and Milisav [79] reported that both NR and NMN, as vitamin B3 forms and NAD+ precursors were not detected to be cancerogenic. Radenkovic and Verdin [80] also have stated that since no study has reported rigorous side effects and they have been in use for many years, therapeutic interventions including NMN, NR, vitamin B3 and niacin supplementation, which increase NAD+ levels, are likely to be fairly safe for the human use.
Radenkovic and Verdin [80] further explained that side effects of long-term NAD upregulation are complicated to identify and quantify, but they still can exist. In addition, it was illustrated that prolonged high dose of vitamin B3 compounds, including NMN may possibly have long-term side effects. According to Rolfe [68], NAD upregulation has the possibility to make worse the senescence-associated secretory phenotype (SASP) generated by senescent cells in aged tissues. A suggested mechanism to cause this side effect was the suppression of 5′ adenosine monophosphate-activated protein kinase and tumor protein p53, directing to stimulate nuclear factor kappa B protein transcription factor through p38 mitogen-activated protein kinases and raised expression of inflammatory cytokines. The cellular senescence burden enlarged with aging, while the produced ASAP was a responsible factor for different age-related pathologies [81].
However, evidences to support these potential side effects of long-term NMN usage and NAD upregulation are lacking. In fact, the usage of anti-aging interventions is expected to be initiated at comparatively younger and healthier age than very old age. Therefore, identification and quantification of adverse effects and challenges of long-term utilisation of anti-aging health products is extremely essential and critical since these anti-aging products are apparently used for a long time. According to Yu et al. [82], NMN treatment (400 mg/kg) obstructed the exercise-induced benefits of a mouse model of diet-induced obesity such as reduced hepatic triglyceride accumulation, glucose stimulated insulin secretion from islets and glucose tolerance. This finding should be further investigated thoroughly since the exercise is one of the key components for maintaining health and wellbeing of the elderly.
Conclusions
NMN is a precursor of NAD+ and an intermediate of NAD+ biosynthesis, which is achieved through three pathways. NMN is an intermediate by-product in two of them. NAD+ levels in the body are depleted with aging as a result of activities of NAD+ consuming enzymes. Depletion of NAD+ level is associated with downregulation of energy production in mitochondria, increasing oxidative stress, DNA damage, cognitive impairments and inflammatory diseases. NMN, as the precursor of NAD+, has been seen to likely reverse these age-related complications and slow down the rate of aging by enhancing NAD+ levels in the body.
Many studies have been done to explore NMN’s anti-aging effects in various cells and tissues. Most of the works have been done in vitro or in animal models. However, published reports about NMN’s long-term safety and clinical efficacy of anti-aging effects in humans are scarce. From the above review, it can be seen that only very few pre-clinical and clinical studies have been conducted to investigate the safety of long-term administration of NMN. A few more human clinical trials are being conducted to evaluate the safety concerns of NMN supplementation and the outcomes are yet to be available.
However, many NMN anti-aging health products are already available in the market and manufacturers are aggressively marketing the products using in vitro and in vivo results from the literature. Therefore, the first priority should be to establish toxicology, pharmacology and safety profiles of NMN in humans, including healthy and diseased. For NMN’s anti-aging efficacy, the most feasible route to obtain data will probably through long-term follow-ups of people who consume NMN regularly. Such research should be supported by NMN manufacturers as they have the moral responsibility to provide efficacy results of their products.
Funding
Funding support from Education New Zealand, New Zealand-China Tripartite Research Collaboration Fund – AUT 13772 to Professors Jun Lu, Jinyao Li, Tianlei Ying and Baohong Zhang; Professor Jun Lu also has received funding support from EZZ Life Science Holdings Pty Ltd.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank Professor Terrence Madhujith, Professor of Food Science and Technology & Department Chair, Department of Food Science and Technology, Faculty of Agriculture, University of Peradeniya, Peradeniya, Sri Lanka, for proofreading this manuscript.
References
United Nations (UN), Department of Economic and Social Affairs, Population Division. World population ageing 2019: highlights (ST/ESA/SER.A/430). New York, USA: United Nations; 2019.
I.M. Rea, D.S. Gibson, V. McGilligan, S.E. McNerlan, H.D. Alexander, O.A. Ross
Age and age-related diseases: role of inflammation triggers and cytokines
Front Immunol, 9 (2018), p. 586
View PDFView Record in ScopusGoogle Scholar
E. Diamanti-Kandarakis, M. Dattilo, D. Macut, L. Duntas, E.S. Gonos, D.G. Goulis, et al.
Aging and anti-aging: a combo-endocrinology overview
Eur J Endocrinol, 176 (2017), pp. 283-308
N.R. Reisman
Anti-aging medicine: the legal issues: legal issues associated with the current and future practice of anti-aging medicine
J Gerontol A Biol Sci Med Sci, 59 (7) (2004), pp. B674-B681
View PDFCrossRefGoogle Scholar
T. Yamamoto, J. Byun, P. Zhai, Y. Ikeda, S. Oka, J. Sadoshima
Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion
PLoS ONE, 9 (6) (2014), p. e98972
View PDFCrossRefView Record in ScopusGoogle Scholar
P. Bieganowski, C. Brenner
Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans
Cell, 117 (4) (2004), pp. 495-502
Download PDFView Record in ScopusGoogle Scholar
G.C. Marinescu, R. Popescu, A. Dinischiotu
Size exclusion chromatography method for purification of nicotinamide mononucleotide (NMN) from bacterial cells
Sci Rep, 8 (1) (2018), pp. 1-11
View Record in ScopusGoogle Scholar
S.E. Poddar, E.K. Sifat, S. Haque, N.A. Nahid, S. Chowdhury, I. Mehedi
Nicotinamide mononucleotide: exploration of diverse therapeutic applications of a potential molecule
Biomolecules, 9 (1) (2019), pp. 34-49
View PDFCrossRefView Record in ScopusGoogle Scholar
National Centre for Biotechnology Information (NCBI). PubChem Compound Summary for CID 14180, Nicotinamide mononucleotide. [cited 2020 Sep 22]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Nicotinamide-mononucleotide.
K.F. Mills, S. Yoshida, L.R. Stein, A. Grozio, S. Kubota, Y. Sasaki, et al.
Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice
Cell Metab, 24 (6) (2016), pp. 795-806
Download PDFView Record in ScopusGoogle Scholar
Virtual Computational Chemistry Laboratory [homepage on the Internet]. ALOGPS 2.1. 2001–2016. [updated 2016; cited 2021 Aug 01]. Aqueous solubility calculation. Available from: http://www.vcclab.org.
Y. Hou, S. Lautrup, S. Cordonnier, Y. Wang, D.L. Croteau, E. Zavala, et al.
NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency
Proc Natl Acad Sci U S A, 115 (8) (2018), pp. E1876-E1885
View PDFCrossRefView Record in ScopusGoogle Scholar
K. Okabe, K. Yaku, K. Tobe, T. Nakagawa
Implications of altered NAD metabolism in metabolic disorders
J Biomed Sci, 26 (1) (2019), pp. 34-47
View PDFView Record in ScopusGoogle Scholar
G.C. Marinescu, R.G. Popescu, G. Stoian, A. Dinischiotu
β-nicotinamide mononucleotide (NMN) production in Escherichia coli
Sci Rep, 8 (1) (2018), pp. 1-11
View Record in ScopusGoogle Scholar
W. Hong, F. Mo, Z. Zhang, M. Huang, X. Wei
Nicotinamide mononucleotide: a promising molecule for therapy of diverse diseases by Targeting NAD+ metabolism
Front Cell Dev Biol, 8 (2020), p. 246
View PDFView Record in ScopusGoogle Scholar
L. Mouchiroud, R.H. Houtkooper, N. Moullan, E. Katsyuba, D. Ryu, C. Cantó, et al.
The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling
Cell, 154 (2) (2013), pp. 430-441
Download PDFView Record in ScopusGoogle Scholar
E.F. Fang, S. Lautrup, Y. Hou, T.G. Demarest, D.L. Croteau, M.P. Mattson, et al.
NAD+ in aging: molecular mechanisms and translational implications
Trends Mol Med, 23 (10) (2017), pp. 899-916
Download PDFView Record in ScopusGoogle Scholar
J. Camacho-Pereira, M.G. Tarragó, C.C. Chini, V. Nin, C. Escande, G.M. Warner, et al.
CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism
Cell Metab, 23 (6) (2016), pp. 1127-1139
Download PDFView Record in ScopusGoogle Scholar
J.A. Hall, J.E. Dominy, Y. Lee, P. Puigserver
The sirtuin family’s role in aging and age-associated pathologies
J Clin Invest, 123 (3) (2013), pp. 973-979
View PDFView Record in ScopusGoogle Scholar
C. Cantó, K.J. Menzies, J. Auwerx
NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus
Cell Metab, 22 (1) (2015), pp. 31-53
Download PDFView Record in ScopusGoogle Scholar
S.I. Imai
Dissecting systemic control of metabolism and aging in the NAD World: the importance of SIRT1 and NAMPT-mediated NAD biosynthesis
FEBS Lett, 585 (11) (2011), pp. 1657-1662
Download PDFCrossRefView Record in ScopusGoogle Scholar
Y. Yang, A.A. Sauve
NAD+ metabolism: bioenergetics, signaling and manipulation for therapy
Biochim Biophys Acta Proteins Proteom, 1864 (12) (2016), pp. 1787-1800
Download PDFView Record in ScopusGoogle Scholar
J. Yoshino, K.F. Mills, M.J. Yoon, S.I. Imai
Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet-and age-induced diabetes in mice
Cell Metab, 14 (4) (2011), pp. 528-536
Download PDFView Record in ScopusGoogle Scholar
A.P. Gomes, N.L. Price, A.J. Ling, J.J. Moslehi, M.K. Montgomery, L. Rajman, et al.
Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging
Cell, 155 (7) (2013), pp. 1624-1638
Download PDFView Record in ScopusGoogle Scholar
C. Cantó, L.Q. Jiang, A.S. Deshmukh, C. Mataki, A. Coste, M. Lagouge, et al.
Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle
Cell Metab, 11 (3) (2010), pp. 213-219
Download PDFView Record in ScopusGoogle Scholar
E. Verdin
NAD+ in aging, metabolism, and neurodegeneration
Science, 350 (6265) (2015), pp. 1208-1213
View PDFCrossRefView Record in ScopusGoogle Scholar
S.I. Imai
A possibility of nutriceuticals as an anti-aging intervention: activation of sirtuins by promoting mammalian NAD biosynthesis
Pharmacol Res, 62 (1) (2010), pp. 42-47
Download PDFView Record in ScopusGoogle Scholar
J.H. Lim, Y.M. Lee, Y.S. Chun, J. Chen, J.E. Kim, J.W. Park
Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1α
Mol Cell, 38 (6) (2010), pp. 864-878
Download PDFView Record in ScopusGoogle Scholar
N.E. De Picciotto, L.B. Gano, L.C. Johnson, C.R. Martens, A.L. Sindler, K.F. Mills, et al.
Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice
Aging Cell, 15 (3) (2016), pp. 522-530
View PDFView Record in ScopusGoogle Scholar
T. Kawamura, N. Mori, K. Shibata
β-Nicotinamide mononucleotide, an anti-aging candidate compound, is retained in the body for longer than nicotinamide in rats
J Nutr Sci Vitaminol, 62 (4) (2016), pp. 272-276
View PDFCrossRefView Record in ScopusGoogle Scholar
Y. Guan, S.R. Wang, X.Z. Huang, Q.H. Xie, Y.Y. Xu, D. Shang, et al.
Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1–dependent manner
J Am Soc Nephrol, 28 (8) (2017), pp. 2337-2352
View PDFView Record in ScopusGoogle Scholar
J. Li, M.S. Bonkowski, S. Moniot, D. Zhang, B.P. Hubbard, A.J. Ling, et al.
A conserved NAD+ binding pocket that regulates protein-protein interactions during aging
Science, 355 (6331) (2017), pp. 1312-1317
View PDFCrossRefView Record in ScopusGoogle Scholar
K. Tsubota
Anti-aging approach for ocular disorders: from dry eye to retinitis pigmentosa and myopia
Nippon Ganka Gakkai Zasshi, 121 (3) (2017), pp. 232-248
View Record in ScopusGoogle Scholar
A. Das, G.X. Huang, M.S. Bonkowski, A. Longchamp, C. Li, M.B. Schultz, et al.
Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging
Cell, 173 (1) (2018), pp. 74-89
View Record in ScopusGoogle Scholar
S. Johnson, D.F. Wozniak, S. Imai
CA1 NAMPT knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves
npj Aging Mech Dis, 4 (1) (2018), pp. 1-12
View PDFCrossRefView Record in ScopusGoogle Scholar
L. Hosseini, F. Farokhi-Sisakht, R. Badalzadeh, A. Khabbaz, J. Mahmoudi, S. Sadigh-Eteghad
Nicotinamide mononucleotide and melatonin alleviate aging-induced cognitive impairment via modulation of mitochondrial function and apoptosis in the prefrontal cortex and hippocampus
Neuroscience, 423 (2019), pp. 29-37
Download PDFView Record in ScopusGoogle Scholar
T. Kiss, C.B. Giles, S. Tarantini, A. Yabluchanskiy, P. Balasubramanian, T. Gautam, et al.
Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects
Geroscience, 41 (4) (2019), pp. 419-439
View PDFCrossRefView Record in ScopusGoogle Scholar
J. Song, J. Li, F. Yang, G. Ning, L. Zhen, L. Wu, et al.
Nicotinamide mononucleotide promotes osteogenesis and reduces adipogenesis by regulating mesenchymal stromal cells via the SIRT1 pathway in aged bone marrow
Cell Death Dis, 10 (5) (2019), pp. 1-12
View PDFCrossRefView Record in ScopusGoogle Scholar
S. Tarantini, M.N. Valcarcel-Ares, P. Toth, A. Yabluchanskiy, Z. Tucsek, T. Kiss, et al.
Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice
Redox Boil, 24 (2019), p. 101192
Download PDFView Record in ScopusGoogle Scholar
L. Hosseini, M.S. Vafaee, R. Badalzadeh
Melatonin and nicotinamide mononucleotide attenuate myocardial ischemia/reperfusion injury via modulation of mitochondrial function and hemodynamic parameters in aged rats
J Cardiovasc Pharmacol Ther, 25 (3) (2020), pp. 240-250
View PDFCrossRefView Record in ScopusGoogle Scholar
T. Kiss, Á. Nyúl-Tóth, P. Balasubramanian, S. Tarantini, C. Ahire, A. Yabluchanskiy, et al.
Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects
GeroScience, 42 (2020), pp. 527-546
View PDFCrossRefView Record in ScopusGoogle Scholar
Y. Miao, Z. Cui, Q. Gao, R. Rui, B. Xiong
Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes
Cell Rep, 32 (5) (2020), p. 107987
Download PDFView Record in ScopusGoogle Scholar
M.J. Bertoldo, D.R. Listijono, W.H.J. Ho, A.H. Riepsamen, D.M. Goss, D. Richani, et al.
NAD+ repletion rescues female fertility during reproductive aging
Cell Rep, 30 (6) (2020), pp. 1670-1681
View PDFView Record in ScopusGoogle Scholar
Fu R, Zhang Q. Inventors; Hoboomlife Bio-Technology Co Ltd, assignee. Use of β-nicotinamide mononucleotide in preparation of anti-aging drugs or health-care products. United States patent application US 15/310,371; 2017 Sep 21. [cited 2020 Oct 25]. Available from: https://patents.google.com/patent/US20170266213A1/en.
D. Wahl, R.M. Anderson, D.G. Le Couteur
Antiaging therapies, cognitive impairment, and dementia
J Gerontol A, 75 (9) (2020), pp. 1643-1652
View PDFCrossRefView Record in ScopusGoogle Scholar
E.F. Fang
Mitophagy and NAD+ inhibit Alzheimer disease
Autophagy, 15 (6) (2019), pp. 1112-1114
View PDFCrossRefView Record in ScopusGoogle Scholar
A.N. Long, K. Owens, A.E. Schlappal, T. Kristian, P.S. Fishman, R.A. Schuh
Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model
BMC Neurol, 15 (1) (2015), pp. 1-14
View PDFCrossRefView Record in ScopusGoogle Scholar
X. Wang, X. Hu, Y. Yang, T. Takata, Y. Sakurai
Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death
Brain Res, 1643 (2016), pp. 1-9
Download PDFCrossRefView Record in ScopusGoogle Scholar
Z. Yao, W. Yang, Z. Gao, P. Jia
Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease
Neurosci Lett, 647 (2017), pp. 133-140
Download PDFView Record in ScopusGoogle Scholar
N. Klimova, A. Long, T. Kristian
Nicotinamide mononucleotide alters mitochondrial dynamics by SIRT3-dependent mechanism in male mice
J Neurosci Res, 97 (8) (2019), pp. 975-990
View PDFCrossRefView Record in ScopusGoogle Scholar
L. Lu, L.E. Tang, W. Wei, Y. Hong, H. Chen, W. Ying, et al.
Nicotinamide mononucleotide improves energy activity and survival rate in an in vitro model of Parkinson's disease
Exp Ther Med, 8 (3) (2014), pp. 943-950
View PDFCrossRefView Record in ScopusGoogle Scholar
E.F. Fang, Y. Hou, S. Lautrup, M.B. Jensen, B. Yang, T. SenGupta, et al.
NAD+ augmentation restores mitophagy and limits accelerated aging in Werner syndrome
Nat Commun, 10 (1) (2019), pp. 1-18
View PDFCrossRefView Record in ScopusGoogle Scholar
M.N. Okur, B. Mao, R. Kimura, S. Haraczy, T. Fitzgerald, K. Edwards-Hollingsworth, et al.
Short-term NAD+ supplementation prevents hearing loss in mouse models of Cockayne syndrome
npj Aging Mech Dis, 6 (1) (2020), pp. 1-10
View PDFView Record in ScopusGoogle Scholar
E.F. Fang, H. Kassahun, D.L. Croteau, M. Scheibye-Knudsen, K. Marosi, H. Lu, et al.
NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair
Cell Metab, 24 (4) (2016), pp. 566-581
Download PDFView Record in ScopusGoogle Scholar
M. Tosato, V. Zamboni, A. Ferrini, M. Cesari
The aging process and potential interventions to extend life expectancy
Clin Interv Aging, 2 (3) (2007), pp. 401-412
View PDFView Record in ScopusGoogle Scholar
B. Cardona
Dangers and dilemmas surrounding the consumption of anti-ageing medicine
B.V. Rodopi (Ed.), Justice for older people, Brill Publishers, Netherlands (2012), pp. 35-45
M.J. Yoshino, B.D. Yoshino, G. Kayser, M.P. Patti, K.F. Franczyk, M. Mills, T. Sindelar, B.W. Pietka, S.I. Patterson, S Klein Imai
Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women
Science (2021), p. eabe9985
View Record in ScopusGoogle Scholar
V. Brower
A nutraceutical a day may keep the doctor away: Consumers are turning increasingly to food supplements to improve wellbeing when pharmaceuticals fail
EMBO Rep, 6 (8) (2005), pp. 708-711
View PDFCrossRefView Record in ScopusGoogle Scholar
L. Rajman, K. Chwalek, D.A. Sinclair
Therapeutic potential of NAD-boosting molecules: the in vivo evidence
Cell Metab, 27 (3) (2018), pp. 529-547
Download PDFView Record in ScopusGoogle Scholar
M. Mehmel, N. Jovanović, U. Spitz
Nicotinamide riboside-the current State of research and therapeutic uses
Nutrients, 12 (6) (2020), p. 1616
View PDFCrossRefView Record in ScopusGoogle Scholar
N. Braidy, J. Berg, J. Clement, F. Khorshidi, A. Poljak, T. Jayasena, et al.
Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: rationale, biochemistry, pharmacokinetics, and outcomes
Antioxid Redox Signal, 30 (2) (2019), pp. 251-294
View PDFCrossRefView Record in ScopusGoogle Scholar
A. Grozio, K.F. Mills, J. Yoshino, S. Bruzzone, G. Sociali, K. Tokizane, et al.
Slc12a8 is a nicotinamide mononucleotide transporter
Nature Metab, 1 (1) (2019), pp. 47-57
View PDFCrossRefView Record in ScopusGoogle Scholar
M. Knip, I.E. Douek, W.P.T. Moore, H.A. Gillmor, A.E.M. McLean, P.J. Bingley, et al.
Safety of high-dose nicotinamide: a review
Diabetologia, 43 (11) (2000), pp. 1337-1345
View PDFView Record in ScopusGoogle Scholar
E.S. Hwang, S.B. Song
Possible adverse effects of high-dose nicotinamide: mechanisms and safety assessment
Biomolecules, 10 (5) (2020), pp. 687-708
View PDFCrossRefView Record in ScopusGoogle Scholar
M.A. Assiri, H.R. Ali, J.O. Marentette, Y. Yun, J. Liu, M.D. Hirschey, et al.
Investigating RNA expression profiles altered by nicotinamide mononucleotide therapy in a chronic model of alcoholic liver disease
Hum Genomics, 13 (1) (2019), pp. 1-13
View Record in ScopusGoogle Scholar
D. Li, Y.J. Tian, J. Guo, W.P. Sun, Y.Z. Lun, M. Guo, et al.
Nicotinamide supplementation induces detrimental metabolic and epigenetic changes in developing rats
Br J Nutr, 110 (12) (2013), pp. 2156-2164
View PDFView Record in ScopusGoogle Scholar
I.F. Harrison, N.M. Powell, D.T. Dexter
The histone deacetylase inhibitor nicotinamide exacerbates neurodegeneration in the lactacystin rat model of Parkinson's disease
J Neurochem, 148 (1) (2019), pp. 136-156
View PDFCrossRefView Record in ScopusGoogle Scholar
H.M. Rolfe
A review of nicotinamide: treatment of skin diseases and potential side effects
J Cosmet Dermatol, 13 (4) (2014), pp. 324-328
View PDFCrossRefView Record in ScopusGoogle Scholar
R.W. Dellinger, S.R. Santos, M. Morris, M. Evans, D. Alminana, L. Guarente, et al.
Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study
npj Aging Mech Dis, 3 (1) (2017), pp. 1-9
View Record in ScopusGoogle Scholar
O.L. Dollerup, B. Christensen, M. Svart, M.S. Schmidt, K. Sulek, S. Ringgaard, et al.
A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects
Am J Clin Nutr, 108 (2) (2018), pp. 343-353
View PDFCrossRefView Record in ScopusGoogle Scholar
W. Shi, M.A. Hegeman, A. Doncheva, M. Bekkenkamp-Grovenstein, V.C. de Boer, J. Keijer
High dose of dietary nicotinamide riboside induces glucose intolerance and white adipose tissue dysfunction in mice fed a mildly obesogenic diet
Nutrients, 11 (10) (2019), p. 2439
View PDFCrossRefView Record in ScopusGoogle Scholar
G.M. Uddin, N.A. Youngson, D.A. Sinclair, M.J. Morris
Head-to-head comparison of short-term treatment with the NAD+ precursor nicotinamide mononucleotide (NMN) and 6 weeks of exercise in obese female mice
Front Pharmacol, 7 (2016), p. 258
View PDFView Record in ScopusGoogle Scholar
D. Conze, C. Brenner, C.L. Kruger
Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults
Sci Rep, 9 (1) (2019), pp. 1-13
View PDFCrossRefGoogle Scholar
C. Cros, H. Cannelle, L. Laganier, A. Grozio, M. Canault
Safety evaluation after acute and sub-chronic oral administration of high purity nicotinamide mononucleotide (NMN-C®) in Sprague-Dawley rats
Food Chem Toxicol, 150 (2021), p. 112060
Download PDFView Record in ScopusGoogle Scholar
D.B. Conze, J. Crespo-Barreto, C.L. Kruger
Safety assessment of nicotinamide riboside, a form of vitamin B3
Hum Exp Toxicol, 35 (11) (2016), pp. 1149-1160
View PDFCrossRefView Record in ScopusGoogle Scholar
K. Tsubota
K, The first human clinical study for NMN has started in Japan
npj Aging Mech Dis, 2 (1) (2016), p. 16021
View PDFView Record in ScopusGoogle Scholar
J. Irie, E. Inagaki, M. Fujita, H. Nakaya, M. Mitsuishi, S. Yamaguchi, et al.
Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men
Endocr J, 67 (2) (2020), pp. 153-160
View PDFCrossRefView Record in ScopusGoogle Scholar
M. Di Stefano, I. Nascimento-Ferreira, G. Orsomando, V. Mori, J. Gilley, R. Brown, et al.
A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration
Cell Death Differ, 22 (5) (2015), pp. 731-742
View PDFCrossRefView Record in ScopusGoogle Scholar
B. Poljsak, I. Milisav
Vitamin B3 forms as precursors to NAD+: are they safe?
Trends Food Sci Technol, 79 (2018), pp. 198-203
Download PDFView Record in ScopusGoogle Scholar
D. Radenkovic, E. Verdin
Clinical evidence for targeting NAD therapeutically
Pharmaceuticals, 13 (9) (2020), pp. 247-263
View PDFCrossRefGoogle Scholar
A.R. Mendelsohn, J.W. Larrick
Interacting NAD+ and cell senescence pathways complicate antiaging therapies
Rejuvenation Res, 22 (3) (2019), pp. 261-266
View PDFCrossRefView Record in ScopusGoogle Scholar
J. Yu, D.R. Laybutt, L.J. Kim, L.E. Quek, L.E. Wu, M.J. Morris, et al.
Exercise-induced benefits on glucose handling in a model of diet-induced obesity are reduced by concurrent nicotinamide mononucleotide
Am J Physiol Endocrinol Metab, 321 (1) (2021), pp. E176-E189

Harshani Nadeeshani received her B.Sc. (Hons) degree in Food and M.Phil. degree in Science and Technology from the University of Peradeniya, Sri Lanka. She works as a graduate research assistant at the National Institute of Fundamental Studies (NIFS), Sri Lanka. She is a PhD candidate at Auckland University of Technology (AUT), New Zealand. Her research interests include nutritional biochemistry, functional and nutritional properties of food and nutritional status and malnutrition of Sri Lankan children. She has received “The Best Oral Presentation” awards in three conferences which held in Sri Lanka on food and nutrition.

Professor Jinyao Li is the Dean of Life Sciences, Xinjiang University. The aims of his research are (1) to develop therapeutic cancer vaccines, especially dendritic cell-based vaccine against cervical cancer; (2) to develop vaccines against autoimmune diseases; (3) to search for compounds from natural products for the treatment of cancer, obesity and diabetes. His technical expertise including flow cytometry, small animal experimentation, in vitro tissue experimentation, molecular biology technology and cell line experimentation.

Dr. Tianlei Ying joined the School of Basic Medical Sciences, Fudan University in 2014 as Professor and Chief of the Antibody Engineering and Drug Discovery Laboratory. In the past 5 years, he has published over 50 papers and co-authored over 10 patents and patent applications. Currently, he is involved in the development of novel antibody fragments of small size and long half-lives. He also identified panels of highly potent fully human mAbs against cancer and infectious diseases. Some of these mAbs are expected to move into the clinic shortly.

Baohong Zhang holds a Ph.D. in Marine Biology from Ocean University of China. She had a post-doctoral position in Shanghai Jiao Tong University. Then she works in SJTU over 16 years. She is currently an Assocciate Professor working mainly on biopharmaceuticals in Engineering Research Center of Cell and Therapeutic Antibody, Ministry of Education, School of Pharmacy, Shanghai Jiao Tong University.

Professor Jun Lu obtained his BSc from East China Normal University and MSc and PhD from the University of Auckland. He established Biomedical Science teaching programme and research laboratory at AUT. His research interest is mainly in nutraceutical products and metabolic disease. He has published more than 120 peer-reviewed journal articles. He has been working on New Zealand seafood products (including mussel, seaweed, paua, fish and clams) to extract bioactive compounds for more than ten years. He is the principal investigator of HVN-funded project mussel-fucoidan as supplemented superfood for the prevention of type 2 diabetes and alleviation of joint pain.
Peer review under responsibility of Cairo University.
© 2021 The Authors. Published by Elsevier B.V. on behalf of Cairo University.
'NMN' 카테고리의 다른 글
| NMN 암치료 화학요법 인지장애 예방 (0) | 2022.02.02 |
|---|---|
| NMN 노화방지 효과 (0) | 2022.02.02 |
| NMN은 혈관노화를 막습니다 (0) | 2022.02.02 |
| NMN 뇌졸중 (0) | 2022.02.02 |
| NAD+ 강화, 뇌허혈 후 미토콘드리아 건강 향상 (0) | 2022.02.02 |





댓글